News center
Contact us
Dongguan Ji kerr automation Technology Co., LTD
Telephone:0769-83328418
Fax:0769-83328428
Email:[email protected]
Address:80 Luxi Road, Xixi Village, Liaobu Town, Dongguan City, Guangdong Province

trade news
Home > News center > trade news
News center > trade news
Domestic microfluidic technology: The IVD industry is the earliest to welcome the harvest period
Date:2020-10-07 browse:314

來源:動脈網2020-01-15 12:29
The birth of microfluidic technology is the pursuit of maximum automation and efficiency by R&D personnel.
In the late 1950s, Professor Richard Feynman, an American Nobel Prize winner in physics,
predicted that future manufacturing technology would develop along the path from large to small.
In 1959, he used semiconductor materials to miniaturize experimental mechanical systems.
This resulted in the world's first Micro-electro-mechanical Systems (MEMS), which became the cornerstone of future microfluidic technology.
From the definition of microfluidic, the advent of real microfluidic technology was in 1990. Manz and Widmer of Ciba-Geigy, Switzerland,
have used MEMS technology to achieve electrophoretic separation on a microchip,
which has been required to be completed in capillary tubes, and proposed the Micro-Total Analytical System for the first time.
i-TAS is what we now know as microfluidic chips.
When Manz and Widmer first experimented with microfluidic, it was to improve analytical capabilities,
but once the actual microfluidic chip concept was proposed, the researchers quickly realized that reducing the size of the device would bring many benefits.
The "micro" of microfluidic refers to the miniaturization of experimental instruments and equipment (the size is tens to hundreds of microns);
"Flow" means that the subject of the test is a fluid (volume of nanoliters to liters); "Control" stands for the control, manipulation,
and handling of fluids on a miniaturized device.
It belongs to a kind of underlying technology, interweaving chemistry, fluid physics, microelectronics,
new materials and other disciplines of knowledge, theoretically speaking, any fluid involved in the experiment,
microfluidic technology should have a place.
The microfluidic chip is the downstream application unit of microfluidic technology,
and the micro-biochemical analysis system is constructed on the surface of the solid chip through MEMS technology,
so as to realize the rapid and accurate processing and detection of inorganic ions, organic substances, proteins,
nucleic acids and other specific target objects. It integrates key steps such as sample processing,
biochemical reaction and result detection that need to be carried out in the laboratory into a small chip,
so it is also known as "laboratory on a chip" in the industry.
Since Manz and Widmer developed on-chip capillary electrophoresis in 1990,
the scientific community and industry have been involved in this emerging field to carry out various microfluidic chip research
and development with capillary electrophoresis as the main application object. Two years later, Agilent, Shimadzu,
Hitachi and other medical device companies have completed the research and development of corresponding microfluidic products/systems and put them into the market.
In 1994, Mike Ramsey, a researcher at Oak Ridge National Laboratory in the United States,
improved the chip capillary electrophoresis sampling method based on the original research of Manz and Widmer, and improved its performance.
In the same year, the world's first International Academic Conference on Micrototal Analytical Systems was held in Enschede,
the Netherlands, and microfluidic chips fully entered the public vision. The following year, Caliper Life Sciences,
the world's first company specializing in microfluidic chip technology, was founded in Massachusetts.
Since the birth of the first microfluidic technology company in 1995,
microfluidic chips have officially opened the road to commercialization and industrialization.
The rapid template copying method of chips PDMS and the soft lithography microvalve/micropump of chips have been proposed successively.
The first commercial instrument of microfluidic chips was jointly launched by Agilent and Galiper in 1999. It is used in the field of biological analysis and clinical analysis.
Microfluidic technology has been developed in foreign countries for ten years,
until the beginning of the 21st century officially entered China, with the gradual rise of in vitro diagnosis (IVD) industry in China,
microfluidic has gradually been known in recent years.
Development technology blossomed
The first shot on the microfluidic track was "Lab on a Chip." The journal was created in 2001 to include articles on microfluidic technology research.
A year later, China ushered in the first academic conference on the theme of microfluidic, that is,
the first National Micrototal Analysis System Conference held in Beijing, to achieve large-scale integration of microfluidic chips.
The research on microfluidic in China started here. Since 2002, a wave of applications for microfluidic related patent products has gradually emerged in China.
By 2012, the annual application number has reached 100, and in 2016, the total number of applications for related patent products has exceeded 600.
The number of patent applications declined somewhat in subsequent years, but remained above 400 a year. At the same time,
the number of papers published by Chinese scientists in the field of microfluidic technology has ranked second in the world,
and the number of patent applications related to microfluidic products is also second only to the United States.
Statistical diagram of the number of microfluid-related papers/patents published/applied annually at home and abroad since the 21st century (arterial network drawing)
With the in-depth study of microfluidic technology year by year,
people also have more in-depth exploration in microfluidic material selection, process technology and other related fields.
From the perspective of microfluidic production materials, semiconductor material silicon material is the preferred material for the preparation of microfluidic chips,
but due to the continuous expansion of microfluidic chip application scenarios, silicon material can not withstand high pressure,
and is not compatible with optical detection technology, so it is abandoned.
Followed by a glass microfluidic chip. Glass material can achieve good electroosmotic properties and optical properties,
theoretically is the perfect microfluidic chip production material, but the glass material is not easy to photolithography and etching,
the production process is complex and time-consuming, and the cost is expensive, resulting in its large-scale promotion.
In contrast, polymer materials reflect the advantages, polymer processing is simple, cheap raw materials, and has good insulation,
high pressure resistance, thermal stability, biocompatibility, gas permeability, low elastic modulus characteristics,
can be widely used in capillary electrophoresis microchips, biochemical reaction chips, a variety of optical detection systems.
The organic polymer represented by polydimethylsiloxane (PDMS) has become a popular material for microfluidic chip fabrication.
In addition, in terms of production process, currently widely used in the production of microfluidic chips are lithography technology,
etching technology, molding method, hot pressing method, LIGA technology, laser ablation technology and soft lithography, etc.,
the specific operation is not described.
Microfluidic manipulation technology is the final step in production,
and the choice of different forces to control the fluid means that the microfluidic chip will end up looking very different. At present,
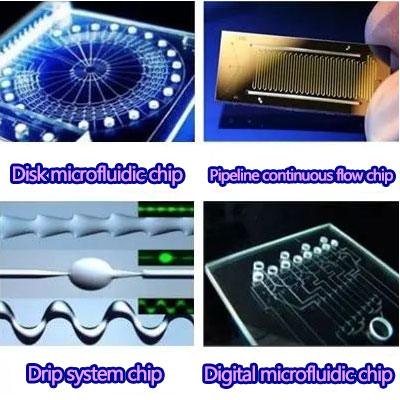
the most common is the disc microfluidic chip, which was proposed by Professor L. ames Lee in 1998 (centrifugal microfluidic CD-ELISA technology).
In addition to disk microfluidic chips, other mainstream microfluidic chips include digital microfluidic chips,
pipeline continuous flow chips, and drip system chips. Because of the different application scenarios,
the "force" controlling the fluid is different, which ultimately leads to the difference in shape.
Digital microfluidic chips are thin and light like paper, and often use external forces such as electromagnetic fields as fluid driving force.
Pipeline continuous flow chips are widely used in the field of CTC cells,
which can selectively identify and capture specific cells in blood and screen them with high throughput,
usually driven by capillary force.
The drop system chip is controlled by discrete microdrops and is suitable for single cell analysis such as digital PCR and second generation sequencing.
In addition, there is a paper-based microfluidic chip that uses paper as a substrate to replace silicon, glass, polymers and other materials,
and the driving force mainly relies on the capillary force of the fibers inside the paper.
Focus on in vitro diagnostics
After more than ten years of development, microfluidic technology has been exploring more application ways from the initial application of capillary electrophoresis.
Because of its strong integration, microfluidic chips can simultaneously process a large number of samples in parallel, with fast analysis,
less energy consumption, low pollution characteristics, appear in biomedical research, drug synthesis and screening, environmental monitoring and protection,
health and quarantine, judicial identification, biological reagent detection and other application scenarios.
In these many application scenarios, microfluidic has a deeper correlation with in vitro diagnosis. As early as 2002,
since the first microfluidic academic conference,
China has provided tens of millions of yuan of financial support every year for relevant companies researching microfluidic
technology to promote the development of domestic microfluidic technology.
On July 28, 2016, The State Council issued the "13th Five-Year Plan for National Science and Technology Innovation",
which clearly proposed that "in vitro diagnostic products should break through key technologies such as microfluidic chips and single molecule detection,
and develop major products such as automatic nucleic acid detection systems."
To develop a number of diagnostic reagents for the early diagnosis and precise treatment of major diseases,
as well as high-precision diagnostic products suitable for primary medical institutions."
Subsequently, the Ministry of Science and Technology issued the "13th Five-Year Plan" Biotechnology Innovation Special Plan,
which clearly included microfluidic chips in a new generation of biodetection technology, and called it a disruptive technology.
The binding of microfluidic and in vitro diagnosis has been certified from the policy level,
and nearly 90% of domestic companies that have developed microfluidic chips have applied them to the field of in vitro diagnosis.
In addition to policy driving, the field of in vitro diagnostics has become the largest part of the microfluidic market segment,
which is inseparable from the rapid development of in vitro diagnostics in recent years.
The compound growth rate of China's IVD industry in 2017 to 2019 reached 18.7%, the IVD market size reached 60.4 billion yuan in 2018,
and the IVD market size is expected to exceed 70 billion yuan in 2019.
The IVD field has driven the innovation of the underlying technology and has become the first industry to land microfluidic technology.
At present, the field of in vitro diagnosis can be divided into immunodiagnosis, biochemical diagnosis, molecular diagnosis, POCT,
blood diagnosis, microbial diagnosis and others according to the proportion of market segments from large to small.
According to the microfluidic chip's micro, high efficiency, low cost and other characteristics,
the proportion of enabling POCT industry is the largest in the IVD subdivision circuit,
and the demand for microfluidic chip in POCT diagnostic equipment is also increasing.
POCT will become the biggest driving force for the development of microfluidic industry.
According to the latest data from Yole analysts, the global microfluidic product market reached 9.98 billion US dollars in 2019,
the microfluidic equipment market reached 3.48 billion US dollars,
and the compound annual growth rate of the microfluidic product market from 2019 to 2024 was as high as 11.7%,
and the compound annual growth rate of the microfluidic product market was 10.8%. It is estimated that by 2024,
the microfluidic product market will reach $17.38 billion, and the microfluidic device market will reach $5.81 billion.
In the face of such a large market, capital will certainly not miss.
Arterial network investigated nearly 50 domestic enterprises involved in the research and development of microfluidic technology,
and counted the number of financing deals and rounds they have obtained since 2015.
(Note: Undisclosed financing information is not included in the statistics, based on the statistical results of the valid information of 26 enterprises.
Since the amount of financing is not disclosed by most companies, the change of financing amount in recent years is not counted.)
Since 2016, an average of more than 10 financing deals per year have been invested in companies involved in microfluidic technology,
mostly in the vicinity of Series A (including pre-A and A+ rounds). In 2019, it is a year of harvest for microfluidic technology,
and four in vitro diagnostic companies involving microfluidic technology have completed financing of about 100 million yuan,
namely Rongzhi Biology, Jingzhu Medical, New Geyuan Biology and Lanyu Biology.
Among them, QuanPLEX, a microfluidic nucleic acid quantitative analysis platform of Rongzhi Biology,
is a microfluidic gene detection platform based on fluorescence quantitative PCR technology,
and three detection applications have been developed. QuanPLEX food-borne pathogen rapid identification system,
QuanPLEX Avian influenza virus detection system and QuanPLEX respiratory pathogen detection system.
We mainly focus on molecular diagnosis in the field of IVD, and have six molecular diagnostic technology platforms: real-time fluorescence quantitative PCR,
first-generation Sanger sequencing, second-generation high-throughput NGS sequencing, Capillary electrophoresis fragment Analysis,
fluorescence in situ hybridization and microfluidic chip. And signed a cooperation agreement with Shanghai Institute of Microsystems and Information Technology,
Chinese Academy of Sciences on microfluidic chip technology;
The company focuses on the development of a new generation of molecular diagnostic tools - mass single cell sequencing,
and launched the first mass single cell sequencing product "mass single cell sequencing" at the beginning of this year. Massive single-cell RNA sequencing products,
including self-developed microfluidic chips, all supporting reagents and the overall solution of biological information analysis software;
The highlight of Lanyu Biology is that it has created an active microfluidic technology platform,
which can give accurate detection results of clinical whole blood samples within 5 minutes. At present,
it has established research and development platforms such as immune rapid diagnosis platform,
handheld electrochemical coagulation platform and nucleic acid automatic rapid diagnosis platform.
Not only that, in 2019, there is another big event in the field of microfluidic technology - micro-point biology is listed on the New Third Board.
Microdot Bio is a company specializing in the R&D,
manufacturing and sales of in vitro point-of-care (POCT) equipment and supporting biological diagnostic testing reagent cards in the medical field.
The company has 13 invention patents in a number of technical fields involved in microfluidic biodetection reagent cards,
mainly mLabs and qLabs technology platforms, mLabs is used to detect heart markers and infection markers. qLabs is the detection of PT/INR,
APTT, PT/APTT and PT/APTT/FIB/TT four types of projects, real-time monitoring of the coagulation status of patients taking medication.

